
(iii) The oxide of calcium is calcium oxide (CaO). (c) Due to large positive charge on the nucleus, the outermost shell is pulled with more force by the nucleus, it moves closer to the nucleus and the size of the atom decreases. (b) Because the atomic number of the elements increases which means that the number of protons and electrons in the atoms increases (the extra electrons being added to the same shell). (a) Thus, size of Ca is smaller than K since on moving from left to right in a period of the periodic table, the size of the atoms decreases.

The following reasons explain why the atomic radius will be larger or smaller than that of potassium with atomic number 19: (ii) Potassium (K) with atomic number 19 comes before Calcium (Ca) having an atomic number 20 in the same period (IV period). (i) It is a metal because it has two electrons in its outermost shell and it easily loses these two electrons to acquire inert gas configuration and form +2 ion. Atomic Structure of Potassium Atomic Radius: 2.77 Atomic Volume: 45.46cm3/mol Covalent Radius: 2.03 Cross Section (Thermal Neutron Capture) a/barns: 2.1. The one-half of the distance between the nuclei of identical atoms that are bonded together is called the atomic radius which increases down the group and decreases across the period.The atomic number of Ca is 20 thus its electronic configuration is 2, 8, 8, 2 The horizontal rows in the periodic table are referred to as the period while the vertical columns are called the groups in the periodic table. Therefore, the correct answer is option D, potassium is larger in atomic radius. group number 17 but atomic radius increases down the group in the periodic table hence chlorine is larger in atomic radius than bromine. The Periodic Table of the Elements (including Atomic Radius). While chlorine and bromine are present in the same group i.e. Similarly, potassium and bromine are present in the same period and as the atomic radius decreases from left to right in a periodic table, therefore, potassium is larger in atomic radius than bromine because potassium is located at the extreme left in the period while bromine is located at the extreme right in the period. Hence, potassium is larger in atomic radius than sodium.
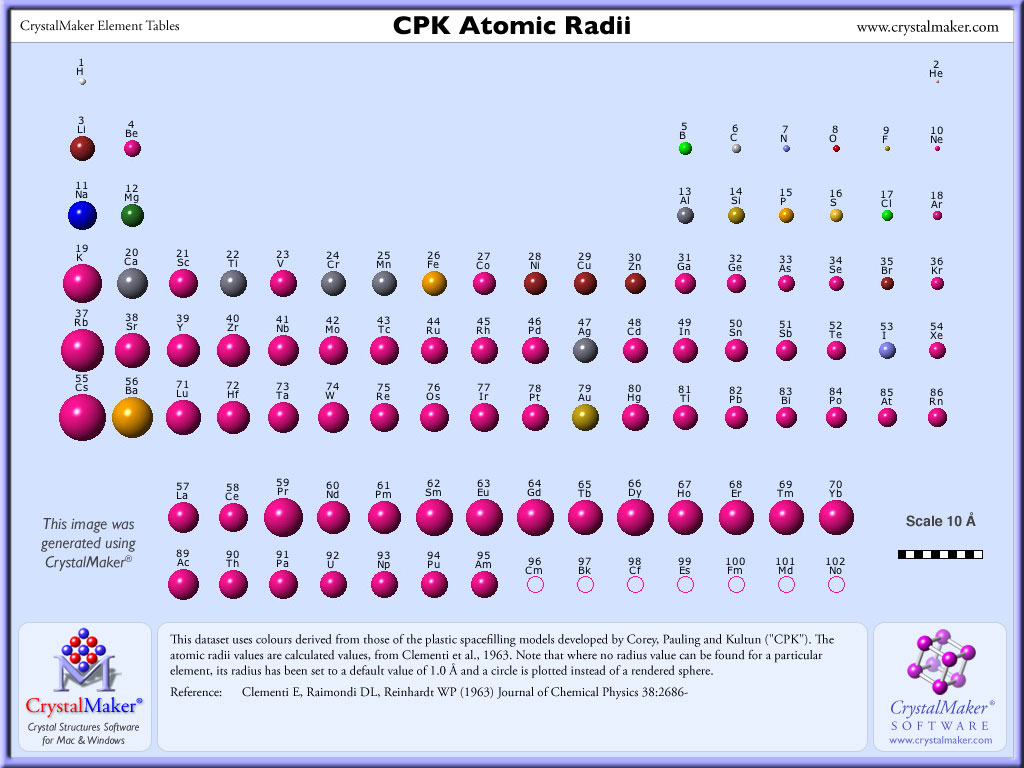
group number one and atomic radius increases down the group in the periodic table. While sodium and potassium are present in the same group in the periodic table i.e. (as we move from above to below in a group ) Atomic size decreases as we move from left to right in a period. Also, atomic size decreases on going from left to right along the period in the periodic table, therefore, sodium is larger in atomic size than chlorine because sodium is present at extreme left in the period and chlorine is present at the extreme right in the period. Answer: Atomic size increases with increase in period number. Potassium comes from a material called Potash. Potassium has an atomic mass of 39.098 atomic mass units. Since sodium and chlorine are located in the same period i.e. CBSE Study Material Textbook Solutions CBSE Notes What Exactly is Potassium Potassium is the first element in the periodic tables fourth period (row).
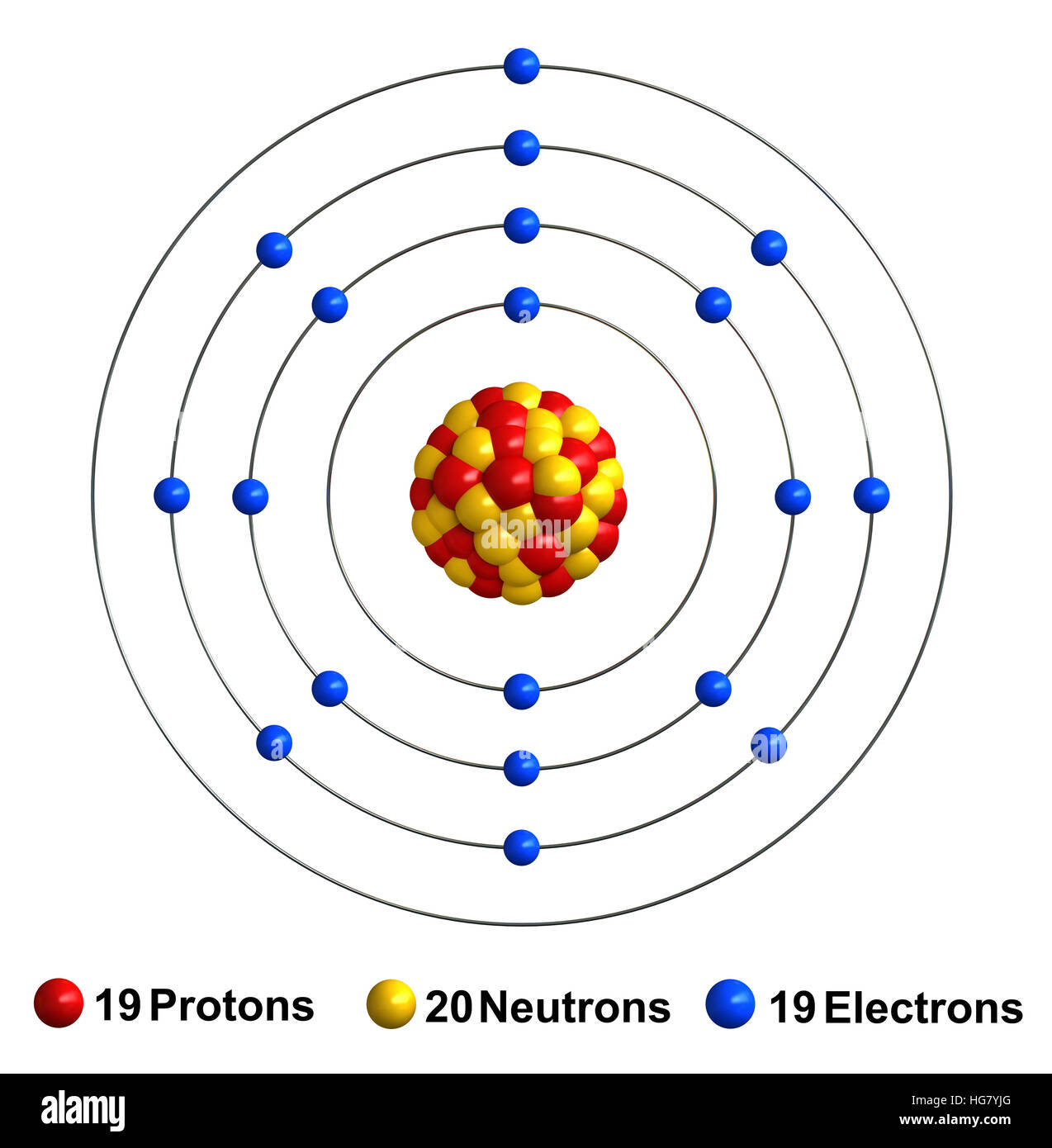
Sodium, potassium belongs to group one of the periodic table and are called alkali earth metals, while chlorine and bromine belong to group seventeen of the periodic table and are called halogens.
Hint: Due to the addition of electrons to the same shell in all atoms, the atomic radius decreases on moving from left to right across the period in a periodic table.


 0 kommentar(er)
0 kommentar(er)
